In the wake of FDA approvals for treating myasthenia gravis (gMG, healthcare providers have the ability to individualize treatment. As these new therapies become adopted, it’s crucial to educate patients and providers about issues such as disease presentation, comorbidities, and patient preferences that may impact treatment decisions.
To support patients and providers, PlatformQ Health, the National Organization for Rare Disorders, and the Myasthenia Gravis Foundation of America worked together to create a robust educational program featuring:
- 60-minute CME activity led by expert clinicians addressing gMG management
- Four 15-minute CME-certified case studies discussing strategies for treatment selection
- A non-accredited 15-minute video showing interactions between a patient/caregiver and providers to help identify the goals and priorities of patients and families
- A non-accredited PDF with key questions clinicians can ask patients to support shared decision-making
- A social media campaign to share micro-learning videos that complement the program
Program outcomes were shared at the 2023 American Association of Neuromuscular & Electrodiagnostic Medicine annual meeting.
Click here to download the full poster.
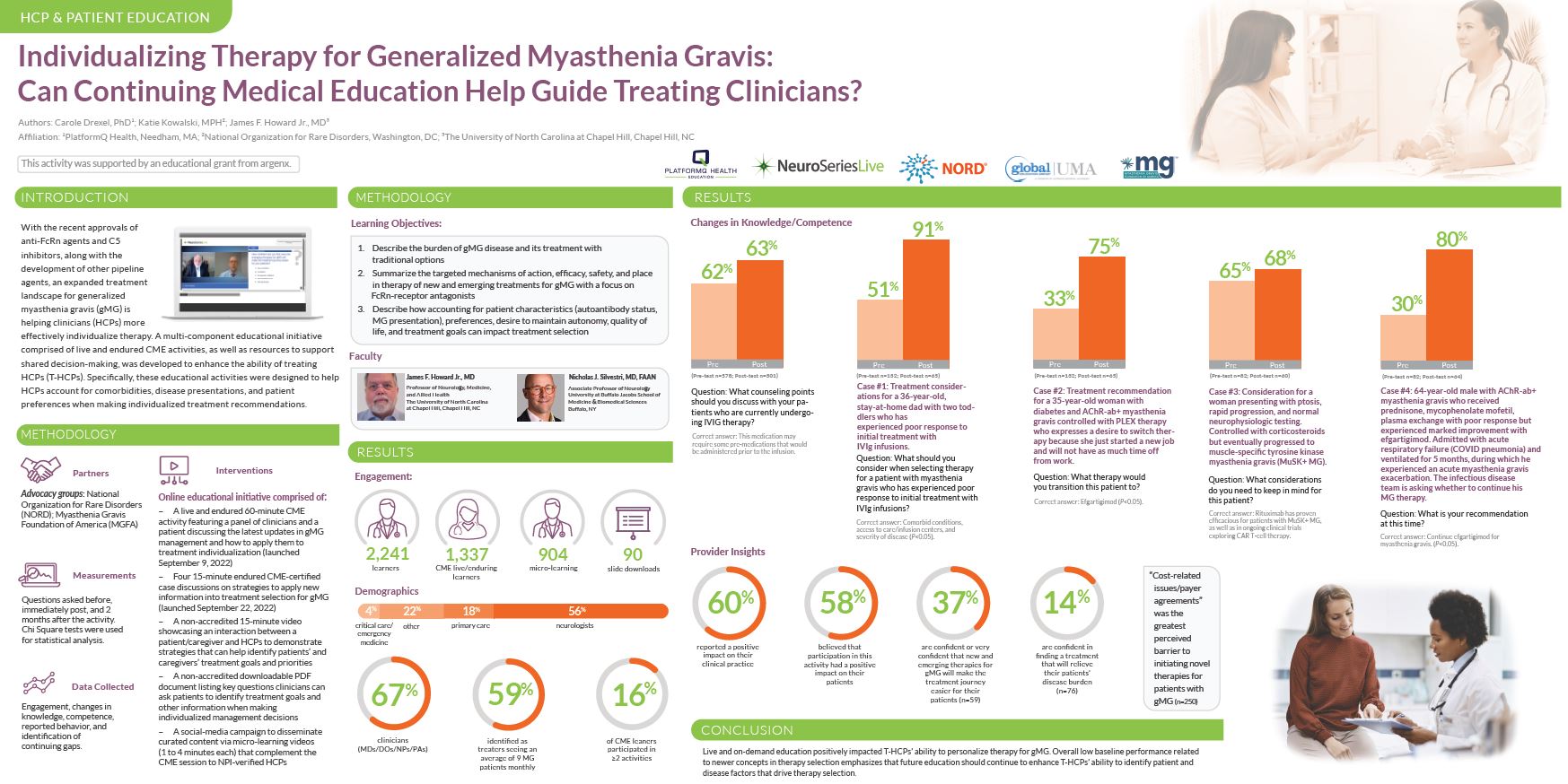
Notably, 2,241 learners participated in the program, including 67% of clinicians. The learners were highly engaged, with 16% participating in more than 2 activities.
Changes in knowledge/competence were significant after completion of the program, with pre- and post-session questions demonstrating notable increases in knowledge on topics such as IVIG therapy, transitioning therapies, and disease progression. 60% of learners reported that the programs had a positive impact on their clinical practice, and 58% believe that participation in the activity had a positive impact on their patients.
These insights and outcomes from these sessions highlight the low initial baseline knowledge related to these therapies and the impact that education can have to improve patient experience and outcomes.